What are neutralizing antibodies?
Neutralizing antibodies are protective proteins that your body makes after a natural infection with SARS-CoV-2, the virus that causes COVID-19. Neutralizing antibodies are also made after vaccination against SARS-CoV-2.
What do neutralizing antibodies do?
The presence of neutralizing antibodies has been shown to block infection from SARS-CoV-2, reduce the severity of disease, and decrease the chance of passing COVID-19 to other individuals.
What is the purpose of this study?
The purpose of this study is to understand if learning your level of neutralizing antibodies changes your perception of protective measures against COVID-19 (like wearing a mask, vaccinations, and booster shots).
What will I be doing in this study?
- You will be asked to complete an online baseline questionnaire about your feelings on COVID-19 and protective measures (like wearing a mask, vaccinations, and booster shots). You will also provide what is called a finger stick dried blood spot sample for the testing of neutralizing antibodies using an at-home collection kit called SeroSure. This kit will be provided to you free of charge along with a pre-paid, pre-addressed return envelope to send the completed SeroSure kit back to the Sponsor’s lab for testing. The SeroSure test is investigational, and the U.S. Food and Drug Administration (FDA) has not determined it safe or effective.
- The SeroSure test result will be returned to you.
- Once you have your test result you will be asked to complete a second online questionnaire about your perceptions of protective measures against COVID-19.
- After 30 days we will follow-up with you to answer a final online questionnaire.
- If you have received any doses of COVID-19 vaccine during the study, we will ask you to upload an image of your vaccine card.
How long will I participate in the study?
The length of your participation (including the time between when you provide the sample and receive the test result) will be approximately two (2) months.
How long will it take me to get my test results?
We hope to get the results of the SeroSure test back to you in 2-4 weeks, although it may take longer in some cases.
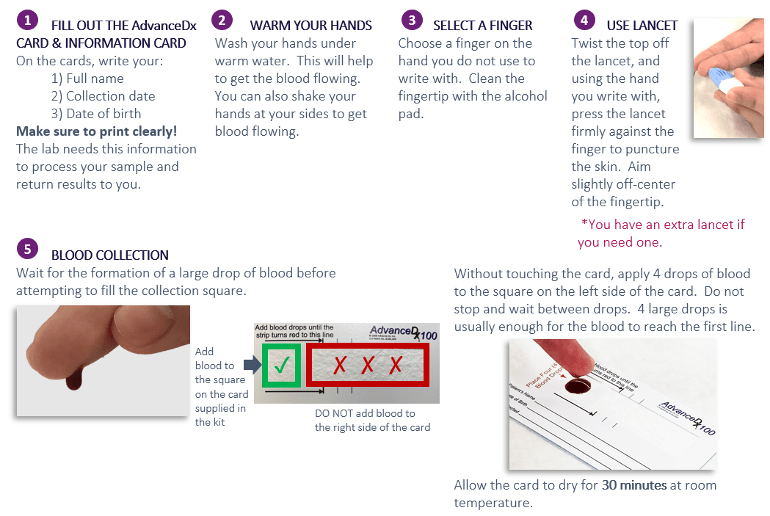
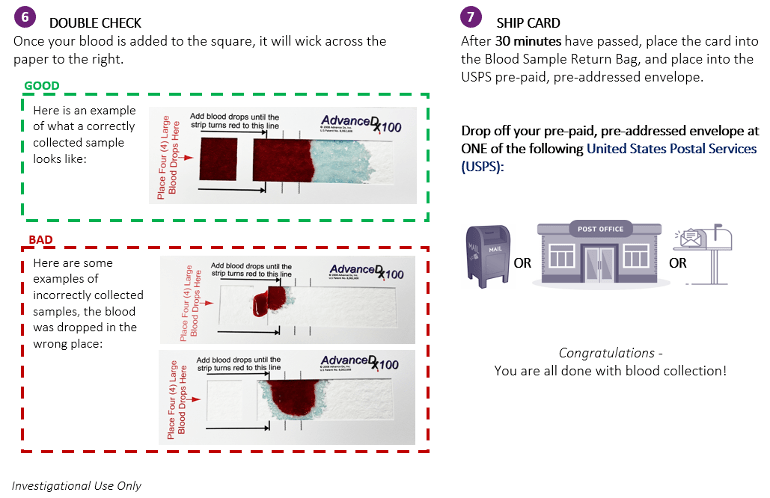
How do I use the test kit?
If you would like to see a short video on what a fingerstick collection is, please go to this link: https://bit.ly/blood-collection or scan this QR code with your phone camera:

My SeroSure Neutralizing Antibody test result was Negative. What does that mean?
Your SeroSure test result is NEGATIVE. This means that you have less neutralizing antibodies than a recently, fully vaccinated individual. You should not assume that you are as protected as a recently, fully vaccinated individual.
Neutralizing antibodies are made by your body after vaccination against SARS-CoV-2 or after a natural infection. Neutralizing antibodies are proteins that your body makes to protect you from COVID-19. The presence of neutralizing antibodies has been shown to block infection from SARS-CoV-2, reduce the severity of disease, and decrease the chance of passing COVID-19 to other individuals.
Recently, fully vaccinated individuals* are largely protected from severe COVID-19 disease because vaccines cause the body to make neutralizing antibodies. Neutralizing antibodies can also be made in response to natural infection. While the actual amount of neutralizing antibodies produced by your body following infection or vaccination cannot be predicted, it can be measured. The SeroSure test is designed to tell you if your level of neutralizing antibodies is the same as or less than a fully vaccinated individual.
Results are for Investigational Use Only and should not be used as part of a clinical diagnosis or patient management algorithm. The performance characteristics of this test have not been established. Results of the SeroSure test should not be used to diagnose or exclude SARS-CoV-2 infection.
*Recently, fully vaccinated individuals are those who have received 2 doses of either the Moderna or the Pfizer vaccine within the first 57 days after initial vaccination, or one dose of the Johnson & Johnson Janssen vaccine within the first 57 days of vaccination.
My SeroSure Neutralizing Antibody test result was Positive. What does that mean?
Your SeroSure test result is POSITIVE. This means that you have a similar amount of neutralizing antibodies as compared to a recently, fully vaccinated individual. It may be reasonable for you to assume that you are as protected as a recently, fully vaccinated individual. Recently, fully vaccinated individuals may still become infected; however, they are less likely to be hospitalized or die than an unvaccinated individual.
Neutralizing antibodies are made by your body after vaccination against SARS-CoV-2 or after a natural infection. Neutralizing antibodies are proteins that your body makes to protect you from COVID-19. The presence of neutralizing antibodies has been shown to block infection from SARS-CoV-2, reduce the severity of disease, and decrease the chance of passing COVID-19 to other individuals.
Recently, fully vaccinated individuals* are largely protected from severe COVID-19 disease because vaccines cause the body to make neutralizing antibodies. However, neutralizing antibodies can also be made in response to natural infection. While the actual amount of neutralizing antibodies produced by your body following infection or vaccination cannot be predicted, it can be measured. The SeroSure test is designed to tell you if your level of neutralizing antibodies that are the same as or less than a recently, vaccinated individual.
*Recently, fully vaccinated individuals are those who have received 2 doses of either the Moderna or the Pfizer vaccine within the first 57 days after initial vaccination, or one dose of the Johnson & Johnson Janssen vaccine within the first 57 days of vaccination.
How will you use my data?
Your blood samples will be tested for neutralizing antibodies and then stored for up to 5 years and may be used for other COVID-19 related research.
Any future studies using your samples and information must be approved by an Institutional Review Board (IRB). Approval from an Institutional Review Board makes sure the proposed studies are ethical and comply with local and federal rules.
Are there any benefits to participating in this study?
There are no direct benefits to you of participating in this study.
Are there any risks to participating in this study?
A risk of participation from this study is discomfort or mild pain from the finger prick blood draw. The SeroSure test used to determine your levels of neutralizing antibodies is for Investigational Use Only and should not be used as part of clinical management.
Is my personal information kept private?
Efforts will be made to limit the use and disclosure of your personal information, including research study records, to people who have a need to review this information. We cannot promise complete secrecy. Organizations that may inspect and copy your information include the Institutional Review Board (IRB), the study sponsor NoniGenex, government agencies such as the U.S. Food and Drug Administration (FDA); and other representatives of Northwestern University, such as the Office for Human Research Protections (OHRP).
Who can I talk to?
If you have questions, concerns, or complaints, you may contact the project staff at SeroSure@northwestern.edu.
Will participating in this study cost me money?
The SeroSure neutralizing antibody test is free to you. There is no cost to you for participating in this study.
Will I be compensated for participating in this study?
There is no compensation for participating in this study.
